-
Nearly 7 in 10 healthcare professionals (68.1%) who work with pharmaceutical companies on non-R&D collaborations have agreed to be named on Disclosure UK
-
ABPI launch new gateway to patient organisation disclosure information on Disclosure UK.
We are pleased that the proportion of healthcare professionals named on Disclosure UK is at its highest ever, but despite guidance from the NHS on managing conflicts of interest there is still work to do to encourage more people to do so. The patient organisation gateway launched today will be built up over time, and we hope it will be an additional and valuable resource for information on patient organisation relationships, as part of our ongoing commitment to transparency. ABPI Chief Executive Richard Torbett
Today’s Disclosure UK data demonstrates companies’ commitment to invest in the UK, and to work collaboratively with the NHS and patient organisations. Whether we’re partnering with the NHS on a clinical trial, or funding a medical educational event, ultimately all our work is to help contribute to better care for patients. We hope healthcare professionals will recognise the value of transparency in the work we do together and continue to be named on Disclosure UK. ABPI President Ben Osborn
The latest information detailing ‘transfers of value’ made to healthcare organisations (HCOs) and healthcare professionals (HCPs) in 2020 by pharmaceutical companies is published today on Disclosure UK by the Association of the British Pharmaceutical Industry (ABPI).
The figures show that in 2020, an estimated 68.1% of healthcare professionals (HCPs) agreed for the transfers of value they received from pharmaceutical companies for non-R&D* collaborations to be made public on Disclosure UK, compared to 55.9% in 2019 and 57.2% in 2018.
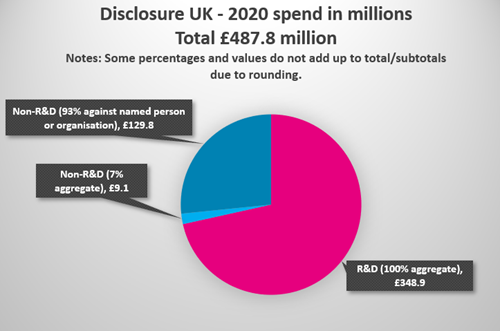
In total, £138.9 million was spent on non-R&D collaborations in 2020 with HCPs and HCOs, of which 93.4% is disclosed against a named person or healthcare organisation. (86.4% for 2019, 83.1% for 2018).
Alongside publication of the 2020 HCP/HCO data, the ABPI has launched a ‘patient organisation gateway’. Pharmaceutical companies have been required to disclose information about relationships with patient organisations on their websites since 2006.
The new gateway is a collection of hyperlinks which enable visitors to the Disclosure UK database to find and review patient organisation disclosures on individual company websites. This additional resource is part of industry’s ongoing commitment to promote transparency.
ABPI Chief Executive Richard Torbett said:
“Partnerships and collaborations are vital in researching and developing the treatments and vaccines that people need. The pandemic has given the world the strongest possible evidence of that. The work that companies and healthcare professionals carry out is vital to helping discover the treatments of the future.
“We are pleased that the proportion of healthcare professionals named on Disclosure UK is at its highest ever, but despite guidance from the NHS on managing conflicts of interest there is still work to do to encourage more people to do so.
“The patient organisation gateway launched today will be built up over time, and we hope it will be an additional and valuable resource for information on patient organisation relationships, as part of our ongoing commitment to transparency.”
Today’s HCP/HCO data shows:
- Pharmaceutical companies spent £487.8 million with HCPs and HCOs in 2020, compared with £542.9 million for 2019.
- £348.9 million of this was spent on R&D*, compared with £381.9 million in 2019.
- £138.9 million of this was spent on non-R&D* collaborations with HCOs and HCPs in 2020, vs £160.9 million in 2019, a 13.7% decrease.
The non-R&D money was spent on the following categories:
- Registration fees – £1.9 million (vs £3.6 million in 2019)
- Sponsorship agreements with HCOs/3rd parties – £24.5 million (£34.1 million)
- Travel and accommodation – £1.5 million (£10.4 million)
- Donations and grants to HCOs – £53.0 million (£44.2 million)
- Fees – £52.0 million (£56.6 million)
- Related expenses agreed in the fee for services or consultancy contract – £2.2 million (£7.5 million)
- Joint Working – £3.9 million (£4.6 million)
The data shows that in 2020, pharmaceutical companies worked with fewer HCPs and HCOs compared with 2019. This is not unexpected given the effect of the pandemic in 2020 and is also reflected in a decrease of values across all but one of the non-R&D categories when compared with 2019 data.
The non-R&D category which increased in value during 2020 is for donations and grants to HCOs. An example of this type of support was from Servier Laboratories Ltd, who supplied grants for hospitals to buy iPads for patients to keep in touch with their relatives at a time when visitors were not allowed during the pandemic.
The ABPI has seen a small but growing number of companies moving to the legal basis of ‘Legitimate Interests’, instead of ‘Consent’, to manage their HCP disclosure commitments.
For information on what this means, see our factsheet available from the Disclosure UK resources.
ABPI President Ben Osborn said:
“Today’s Disclosure UK data demonstrates companies’ commitment to invest in the UK, and to work collaboratively with the NHS and patient organisations.
“Whether we’re partnering with the NHS on a clinical trial, or funding a medical educational event, ultimately all our work is to help contribute to better care for patients. We hope healthcare professionals will recognise the value of transparency in the work we do together and continue to be named on Disclosure UK.”
The ABPI’s 2021 Code of Practice for the pharmaceutical industry comes into force from 1 July 2021 and introduces an evolution of disclosure requirements for the pharmaceutical industry It now includes an additional requirement to disclose payments in aggregate for certain contracted services paid to members of the public, including patients and journalists from 2022, to be disclosed in 2023. This will be done on company websites.
Disclosure UK is the public, searchable database publishing ‘transfers of value’ (payments and benefits in kind) from pharmaceutical companies to UK healthcare professionals and organisations. It is part of pharmaceutical industry transparency requirements across Europe.
Last modified: 28 June 2022
Last reviewed: 28 June 2022