- For the third year, 2021 data shows an increased percentage rate of health professionals agreeing to be named on Disclosure UK who work with pharmaceutical companies on non-R&D collaborations - an estimated 72.6% or more than 7 in 10.
- R&D spend increased by 16% to £405 million in 2021
Partnerships between industry and the NHS are essential – they help develop new medicines and vaccines, improve care for patients and benefit the NHS. Richard Torbett, Chief Executive, ABPI
The latest information detailing ‘transfers of value’ made to healthcare organisations (HCOs) and health professionals (HCPs) in 2021 by pharmaceutical companies is published today on Disclosure UK by the Association of the British Pharmaceutical Industry (ABPI).
The figures show that in 2021, an estimated 72.6% of health professionals (HCPs) agreed for the transfers of value they received from pharmaceutical companies for non-R&D* collaborations to be made public on Disclosure UK, an increase compared to 68.1% in 2020 and 55.9% in 2019.
A total of £405 million was disclosed by companies for R&D* collaborations with HCPs and HCOs in 2021 (£349m in 2020, £382m in 2019). This increase puts R&D activity back on the rising trend seen up to 2019, prior to the pandemic.
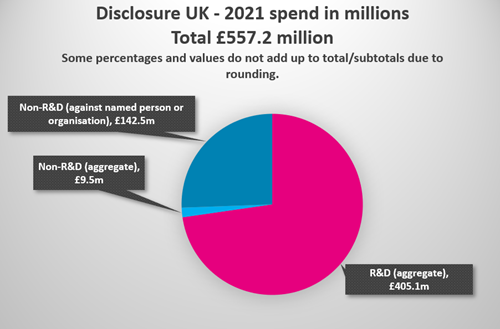
In total, £152 million was spent on non-R&D collaborations in 2021 with HCPs and HCOs, which is down on the amount seen prior to the pandemic. The estimated number of individual health professionals receiving non-R&D values has not returned to pre-pandemic levels. Of the £152 million, 93.8% is disclosed against a named person or healthcare organisation. (93.4% in 2020, 86.4% for 2019).
A total of 139 pharmaceutical companies disclosed 2021 data via Disclosure UK, up from 131 the previous year.
ABPI Chief Executive Richard Torbett said:
“The increase in the estimated percentage rate of health professionals individually named on Disclosure UK, is good news for transparency.
“Partnerships between industry and the NHS are essential – they help develop new medicines and vaccines, improve care for patients and benefit the NHS.
“Of course, we want to improve the transparency of Disclosure UK still further and are working hard to do so. However, the latest data is an encouraging sign that we are on the right track.”
ABPI President Pinder Sahota said:
“Today’s Disclosure UK data shows some fluctuations in different aspects of pharmaceutical company spending that were not unexpected, given the impact of the pandemic and its aftermath on industry and the NHS alike.
“Our focus now is to make sure that non-Covid research and development activity fully recovers after the pandemic, and grows further in the UK, with all the associated benefits for patients, the NHS and the economy.
“The increased rate of health professionals agreeing to be named is very welcome, and we hope to see this continue in future years.”
2021 values
Today’s HCP/HCO data shows:
- Pharmaceutical companies spent £557.2 million with HCPs and HCOs in 2021, compared with £487.8 million in 2020, and £542.9 million in 2019.
- £405.1 million of this was spent on R&D* activities, compared with £348.9 million in 2020, and £381.9 million in 2019.
- £152.0 million of this was spent on non-R&D* collaborations, compared with £138.9 million in 2020, and £160.9 million in 2019
The non-R&D values were disclosed in the following categories:
- Registration fees – £3.0 million (£1.9 million in 2020 and £3.6 million in 2019)
- Sponsorship agreements with HCOs/3rd parties – £32.0 million (£24.5 million in 2020 and £34.1 million in 2019)
- Travel and accommodation – £0.4 million (£1.5 million in 2020 and £10.4 million in 2019)
- Donations and grants to HCOs – £44.0 million (£53.0 million in 2020 and £44.2 million in 2019)
- Contracted services, fees – £65.1 million (£52.0 million in 2020 and £56.6 million in 2019)
- Contracted services, related expenses – £0.4 million (£2.2 million in 2020 and £7.5 million in 2019)
- Collaborative working** – £7.1 million (The numbers are not comparable with previous years as definitions have changed, the nearest comparator is the previous Joint Working category: £3.9 million in 2020 and £4.6 million in 2019)
Decrease in travel and accommodation/expenses
Two categories continue to show significantly lower spends for 2021 in comparison to pre-pandemic years. These are for travel and accommodation, and related expenses as part of contracted services. National lockdowns and movement restrictions continued into 2021 as a result of the pandemic. Since then, the growth of virtual activities and the increase in remote ways of working and focus on sustainability are also factors which will have contributed to the lower spends for these activity types.
Increase in fees
While the overall non-R&D spend is lower in 2021 when compared with 2019, the data shows a 15% increase between 2019 and 2021 specifically in the fees for contracted services with individuals and organisations. We are aware that companies postponed many activities in 2020 due to the pandemic, in part a recognition of the increased pressure the NHS was under. Those activities were then resumed in 2021, in addition to planned 2021 activities.
Legitimate Interests
The ABPI continues to see a small but growing number of pharmaceutical companies moving to the legal basis of ‘Legitimate Interests’, instead of ‘Consent’, to manage their HCP disclosure commitments.
For information on what this means, see our 'What is Legitimate Interests?' factsheet available from the Disclosure UK resources.
Patient organisation gateway
Alongside the annual HCP/HCO data publication, the ABPI’s ‘patient organisation gateway’ has also been updated with 2021 and 2019 information, which joins the 2020 information published last year. These additions bring the patient organisation gateway in-line with the three-years of data published about HCP/HCOs.
The gateway is a collection of hyperlinks which enable visitors to the Disclosure UK database to find and review patient organisation disclosures on individual company websites. Pharmaceutical companies have been required to disclose information about relationships with patient organisations on their websites since 2006.
Last modified: 30 June 2022
Last reviewed: 30 June 2022